Density of Nacl Solution
Add 300mL deionized water to the beaker. The table below gives the density kgL and the corresponding concentration weight of Sodium Chloride NaCl in water at different temperatures in degrees centigrade C.

Variation Of Nacl Aqueous Solutions Density With Temperature And Nacl Download Table
Hence the molality of the solution is 1.
. Molarity moles of soluteliter of solution. Mass of 1 litre of solution 125 gmsml 1000 ml 1 250 gms. Leading life science supplier for your research development or production needs.
Values in the table are consistent with the density of pure water calculated with the IAPWS-95 equation of state. A solution is prepared by dissolving 4g of NaOH to give 500ml of it. Calculate molality of the solution.
Ad Products empowering scientists at every stage helping to deliver scientific breakthroughs. The CO2H2ONaCl solution density increased almost linearly with an increase in the CO2 mass fraction when the NaCl concentration was less than 4 molkg1 and the temperature was lower than 120 C. A linear relationship permits a reliable standard curve to be constructed see Figure 1.
Mass of solutetotal mass of solution100. The influences of pressure temperature CO2 mass fractions and NaCl concentration on the CO2H2ONaCl solution density were analyzed. V volume of water added to make the solution or volume of solvent.
M molkg H 2 O 1. D dfrac m V d V m. Density of 3M solution of NaCl is 125 gmL The mass of the solvent in the solution is.
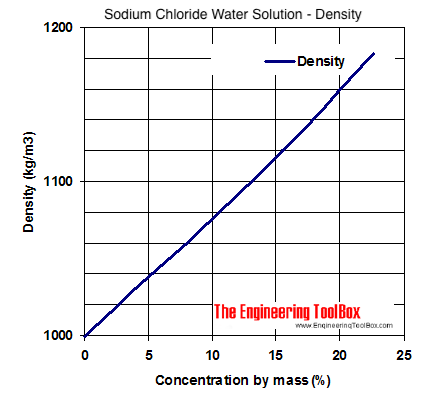
NaCl Solution Preparation You will begin this lab by preparing 1000 mL of a 0182 M solution of NaCl. The density of aqueous NaCl solutions is a nearly-linear function of the NaCl concentration in mass percent. Estimated values of absolute density gcm 3 of aqueous sodium chloride solutions NaCl H 2 O as function of electrolyte molality m and temperature T at pressure p 1 bar.
The density of 3 M solution of NaCl is 125 g mL 1. Input a temperature and density within the range of the table to calculate for concentration or input concentration to calculate for density. Jonathan Pilafas performed on 9102018 Purpose.
D m V. The average density and standard deviation calculations were obtained using Microsoft Excels AVERAGE and STDEVA. Density of sodium chloride is equal to 2 170 kgm³.
10585 1000 585. Molarity is the number of moles of solute NaCl per liter of solution. Weight of solvent Weight of solution Weight of NaCl.
Molarity is the number of moles of solute present in 1 litre solution and molality is the number of moles of solute present in 1 kilogram of solvent. Calculate the molality of the solution. If the sample body has mass m and it occupies volume V then the density of the substance from which it is composed can be calculated using the following formula.
Density of inorganic sodium salts in water is plotted as function of wt molkg water and moll solution. Be aware of the concentration units in the figures. Calculate the molality of the solution.
D density m mass V volume. The objective of this lab is to determine the average density of sodium chloride NaCl at a variety of different concentrations of NaCl in order to find a reliable trend that can be found through graphing. 10585 585 1000g 1 kg.
Therefore m 1 1 1. Preparations and Measurements of 100 NaCl Solution 1. Concentration of NaCl Solutions.
Molecular weight of Na Cl 5844. Sodium chloride is the salt. Sodium chloride NaCl or ClNa CID 5234 - structure chemical names physical and chemical properties classification patents literature biological activities.
You will be following the procedure you used to find the density of pure water. The density of each trial was calculated using Eq. At 20C 68F or 29315K at standard atmospheric pressureIn Imperial or US customary measurement system the density is equal to 135469 pound per cubic foot lbft³ or 1254 ounce per cubic inch ozinch³.
Molality moles of solutekg of water. The mass of the NaCl solution was obtained by subtracting the weight of the empty beaker from the total weighed mass of the beaker plus 10 mL of 15 NaCl solution. Click hereto get an answer to your question The density of 2M solution of sodium chloride NaCl is 113 g moleliter.
A standard curve relates some measurable property such as density to the concentration and is an essential feature. Molarity of NaCl solution - 0 I Solid NaCl will be used in this. Weigh out 333 g of salt and transfer it into a 100ml beaker.
The densities of saturated solutions of NaCl and KCL from 10 degrees to 105 degrees C. With molar masses of 2299 and 3545 gmol respectively 100 g of NaCl contains 3934 g Na and 6066 g Cl. 2M 2mole per litre.
Hence there are 3 5844 gms in 1 Litre of water. Sodium chloride weighs 217 gram per cubic centimeter or 2 170 kilogram per cubic meter ie. Sodium chloride ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d commonly known as salt although sea salt also contains other chemical salts is an ionic compound with the chemical formula NaCl representing a 11 ratio of sodium and chloride ions.
3 Molar solution means there are 3 moles of Na Cl salt in 1 Liter. Density of sodium chloride solutions Objectives Use techniques of measurement for calibrationCalculate the density of a sodium chloride solutionEvaluate. 4 To determine the density of the prepared KI solution.

Sodium Chloride Water Solutions

Variation Of Nacl Aqueous Solutions Density With Temperature And Nacl Download Table

Variation Of Nacl Aqueous Solutions Density With Temperature And Nacl Download Table
No comments for "Density of Nacl Solution"
Post a Comment